Mouse Traps, Hip Implants and E-mails — The Truth Will Out
Building the “better mouse trap” sometimes means producing a product that simply does not work the way it is designed to work. It is a process, sometimes, of trial and error.
If someone (other than the mouse) is seriously injured by that product, it is called negligence.
Building the “better mouse trap” and then hiding or concealing that you know the new mouse trap actually can cause great harm to other than just the mouse is called reckless disregard for the safety of others.
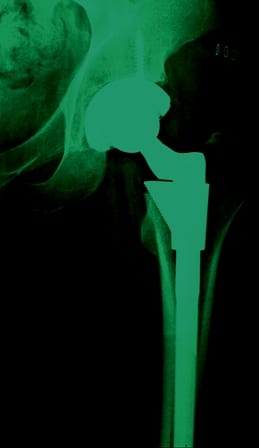 A recently discovered e-mail sets forth what seems to be pretty clear intentions on the part of Johnson & Johnson and its subsidiary, DePuy, to appreciate a dangerous product and not tell anyone about it until they absolutely had to and until they were able to squeak out as much profit as possible.
A recently discovered e-mail sets forth what seems to be pretty clear intentions on the part of Johnson & Johnson and its subsidiary, DePuy, to appreciate a dangerous product and not tell anyone about it until they absolutely had to and until they were able to squeak out as much profit as possible.
The product? Metal-on-metal (MOM) hip implant devices.
The problem? Insufficient testing; inadequate reporting of complications; and continued distribution of a product the manufacturer knew or should have known was defective.
Johnson & Johnson, through its subsidiary Depuy, produced a “metal-on-metal” hip implant that they knew:
- By mid-2009 the FDA refused approval of the implants because studies “showed it had failed prematurely in ‘significant’ numbers, requiring repeat surgeries for patients”.
- They were receiving increasing complaints in the US and abroad of the implants or “companion” implants failing at rising rates.
- That patients were requiring replacement or revision of the devices after only a few years as compared to as many as 15 years or more with other hip implants.
- Failure rates could not have been physician caused, since studies run by surgeons were hand-picked by DePuy (Johnson) and still demonstrated failures.
- Australian medical device regulators were seeing higher failure rates in the device than was acceptable.
The genesis of the problems with devices like the DePuy MOM implants is really two-fold.
First, corporations are answerable to their shareholders on a bottom line profit basis. This motivates any corporation to maximize those profits. As a result, corporations are often motivated in favor of decisions that preserve or increase their bottom line and, sometimes, even if it results in harm to others.
Second, the agency charged with the oversight of products such as metal-on-metal hip implants is the Food & Drug Administration (FDA). Out of necessity, the majority of funding for the FDA comes from the very industries it regulates and it must necessarily cut a fine line between regulation and mediation.
The regulation of medical devices is an important responsibility and the protection of consumers should be the foremost goal; caution should be exercised, even when that caution may cost a manufacturer considerable cost in holding off the marketing of a product.
The reality, however, is the FDA regularly walks a tightrope between consumer protection and biting the hand that feeds you.
[youtube_sc url=”http://www.youtube.com/watch?v=6w90Q3TrkC8&feature=player_embedded”]
Sadly, it is only after 30,000 patients in the US and an unknown number overseas have fallen victim to MOM implants, that the truth is finally seeing the light of day as a result of ongoing litigation.
Share This


