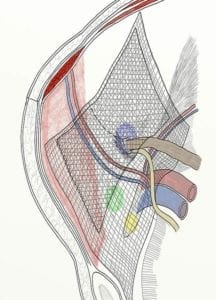
Transvaginal Mesh — An experiment gone wrong
Transvaginal mesh was first developed from surgical mesh used in hernia repair surgeries since the 1950s.
Traditionally, surgical mesh used for hernia repair was constructed of collagen extracted from bovine or human tissue and formed into a mesh that could be implanted in humans. Physicians used the same mesh to treat female stress urinary incontinence and pelvic organ prolapse in the 1970s by cutting it into a different shape. As using mesh in stress urinary incontinence (SUI) and pelvic organ prolapse (POP) surgeries increased in popularity in the late 1990s, medical device companies developed and marketed mesh products in configurations specifically designed to treat these two conditions.
Stress urinary incontinence is defined as losing bladder control caused by physical movement such as lifting, running, coughing, laughing, or sneezing. It can occur during these kinds of activities because of the physical pressure exerted on a bladder weakened by medical or natural events such as childbirth, menopause, pregnancy, surgical procedures, or other conditions. Doctors commonly suggest non-surgical remedies, but sometimes, when surgery was needed, physicians opted for surgical intervention using transvaginal mesh to fix the condition.
Pelvic organ prolapse occurs when a pelvic organ, such as the bladder or uterus, drops (prolapses) from its normal position in the abdomen into a lower portion of the abdomen and may press on other organs. This occurs because the muscles and ligaments holding the pelvic organs may weaken or stretch from childbirth, surgery or the progression of menopause. Most women have some type of prolapse, but not all need treatment. Pelvic organ prolapse can also be caused by chronic constipation causing straining, long-lasting cough, obesity and some types of pelvic organ tumors. Sometimes, the doctor may be able to prescribe exercises, but in other cases, doctors may recommend surgery.
Some history of the vaginal mesh application:
1996: The first manufacturer to produce a vaginal mesh was Boston Scientific with the ProteGen Sling. Other medical device manufacturers quickly developed new products specifically designed to treat stress urinary incontinence. The ProteGen Sling was approved for sale in 1996 by the Food and Drug Administration (FDA) but was recalled just three years later due to safety concerns.
1998: In 1998, Ethicon released the Tension-Free Vaginal Tape (TVT) for SUI. The product was called mesh, tape or sling by medical professionals and laypeople.
2002:The first mesh designed to treat POP was Gynemesh PS, which was released in 2002. It was also designed by Ethicon, a Johnson & Johnson subsidiary.
2008: Due to more than a thousand reports of complications linked to transvaginal mesh devices, the FDA issued the first of two warnings. According to the FDA, women were experiencing infections, pain, mesh erosion, vaginal scarring, pain during intercourse (dyspareunia), and bowel and bladder perforation after undergoing a transvaginal mesh procedure.
In October 2008, the FDA released a statement to doctors and health care providers nationwide, warning of potential complications caused by the implantation of vaginal mesh to treat stress urinary incontinence and pelvic organ prolapse. This statement was based on over 1,000 adverse event reports it had received from doctors and patients. The adverse event reports complications including infection, pain, urinary problems, erosion of the mesh and a recurrence of prolapse and incontinence.
2009 – 2010: In 2010, over 10,000 women underwent vaginal mesh surgery to repair pelvic organ prolapse alone. In an article published in an August 2010 issue of the Obstetrics & Gynecology, researchers reported that scientists had to stop a transvaginal mesh clinical trial early because women implanted with the mesh experienced too many complications. In the trial, which began in 2007, 65 women with pelvic organ prolapse underwent either surgery using the pelvic mesh or a procedure known as colpopexy that uses ligaments to help support the muscles. The trial was cut short in 2009 after researchers found that over 15 percent of the women implanted with the mesh suffered from vaginal mesh erosion within just three months. Vaginal mesh erosion is a potentially serious complication.
2011: A 2011 study released by the Journal of Obstetrics and Gynecology Canada on pelvic mesh procedures stated that “until adequate effectiveness and safety evidence is available, the use of new TVM (transvaginal mesh) devices for prolapse repair should be considered experimental and restricted to use in investigative trials.”
The FDA updated the October 2008 statement in July 2011 with a safety communication alerting doctors to serious and potentially life-threatening complications. In this communication, the FDA also stated that transvaginal mesh procedures were not found to be more effective than traditional surgical options. The data provided by the FDA showed that nearly 10 percent of women who received the vaginal mesh treatment experienced erosion within a year of the implant.
2012: In June 2012, Ethicon voluntarily stopped marketing several of its mesh products, while offering to update marketing and labeling information its other mesh products. This was only after the FDA had received thousands of reports of serious complications because of the products.
The following injuries and/or side effects have been associated with the implantation of transvaginal mesh products:
- Chronic vaginal drainage
- Erosion of the vaginal tissue
- Pain during intercourse
- Perforations of the bowel, bladder or blood vessels
- Reoccurrence of pelvic organ prolapse or stress urinary incontinence
- Urinary problems
- Vaginal bleeding
- Vaginal infections
- Vaginal pain not related to intercourse
- Vaginal scarring
Share This